The Thalidomide Tragedy: A Survivor's Story and a Lasting Warning
The story of Thalidomide is a sobering reminder of the devastating consequences that can arise from unchecked pharmaceutical practices.
A 2012 article from Hankyoreh, a South Korean news outlet, explores this dark chapter through an exclusive interview with Margit Hundemeier, a German survivor of Thalidomide’s catastrophic effects.
Her story, coupled with the broader history of the drug, serves as a powerful cautionary tale about corporate responsibility, regulatory oversight, and the human cost of medical missteps.
A Pill That Changed Lives ForeverIn 1960, Margit Hundemeier’s mother took a single dose of Contergan, a sedative containing Thalidomide, to combat morning sickness during her third month of pregnancy.
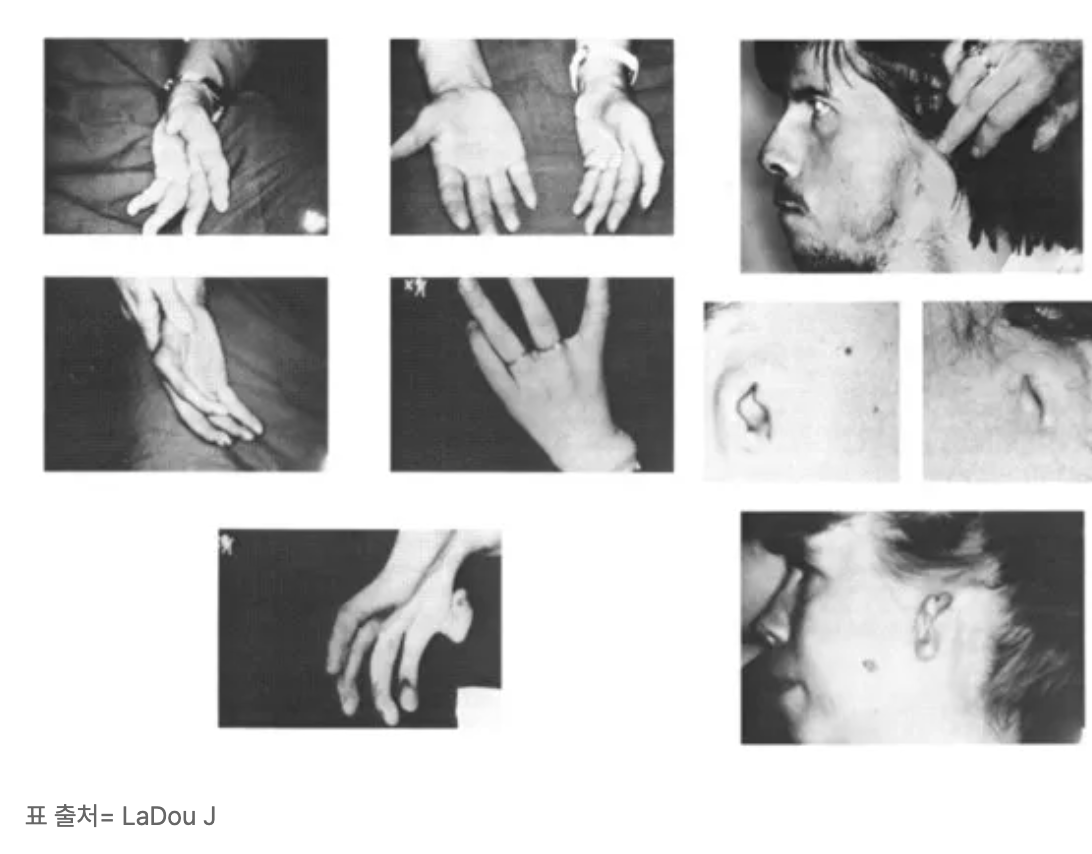
That one pill altered Hundemeier’s life irrevocably. Born without arms, her fingers sprout directly from her shoulders, though her legs and facial features developed normally.
This wasn’t an isolated tragedy. Contergan, manufactured by German pharmaceutical company Grünenthal from 1957 to 1961, was marketed as a cure for ailments like headaches, colds, insomnia, and morning sickness.
Sold in over 50 countries under various names, it was touted as safe—even for pregnant women.
But Thalidomide was anything but safe. When taken between days 21 and 36 of pregnancy, it caused severe birth defects, primarily affecting limb development.
The result was a global catastrophe: approximately 5,000 victims in Germany alone, with another 1,000 worldwide.
Tragically, about half of these individuals have since passed away.
As of 2010, around 2,801 survivors remained, most affected by Contergan, though smaller numbers were harmed by similar drugs from companies like Sweden’s Astra and Britain’s Distillers.
A Fight for JusticeHundemeier’s story is not just one of survival but of resilience and advocacy.
Despite her physical challenges, she pursued education, learned to drive, and became a vocal advocate for Thalidomide victims.
Her efforts focus on holding Grünenthal accountable and raising awareness about the ongoing needs of survivors.
The Hankyoreh article highlights how Grünenthal’s initial denial of responsibility and sluggish response exacerbated the suffering of victims and their families.
Compensation, when it came, was often inadequate, and the company’s failure to fully acknowledge its role remains a sore point for survivors like Hundemeier.
Lessons for TodayThe Thalidomide scandal, one of the worst in medical history, exposed critical flaws in drug testing and regulatory systems.
It spurred significant reforms, including stricter clinical trial protocols and enhanced oversight of pharmaceuticals worldwide.
Yet, as Hundemeier’s interview underscores, the human toll persists. Survivors continue to face physical, emotional, and financial challenges, and the fight for justice is far from over.
This tragedy also resonates with modern environmental and health scandals.
The Hankyoreh piece draws parallels to ongoing issues, urging vigilance to prevent similar disasters.
Whether it’s contaminated water, unchecked pollutants, or rushed medical approvals, the Thalidomide story reminds us that prioritizing profit over safety can have irreversible consequences.
A Call to Remember Margit Hundemeier’s courage in sharing her story serves as both a tribute to Thalidomide survivors and a warning for future generations.
The Hankyoreh article, though published over a decade ago, remains relevant, urging us to prioritize rigorous science, ethical corporate practices, and compassion for those affected by systemic failures.
As Hundemeier’s life demonstrates, resilience can emerge from tragedy, but prevention must always come first.
Source: Hankyoreh, “One Pill’s Curse: The Tragedy of Thalidomide”